母愛研創,閃耀升級雀巢®能恩全護®INFINIPRO® 配方昇華母源免疫精粹7HMOs^,加乘星級雙益生菌+,組成「免疫加乘組合」,科研實證3大免疫加乘功效,配合低敏水解蛋白pHF-W,進一步倍放低敏全護力。



要支持免疫力,理想腸道菌群應以雙歧益生菌為主5。科研實證,「免疫加乘組合」可增加寶寶雙歧益生菌佔比30%1▵及減少壞菌佔比57%1▪,令腸道菌群貼近理想水平+。


珍貴免疫營養(SCFA)可多方面支持免疫力6。
媽媽可為寶寶補充合適的加乘組合,更有效增加寶寶腸道內的珍貴免疫營養。


免疫系統失衡,可增加寶寶過敏風險!
科研實證,「免疫加乘組合」可促進母源免疫平衡▼,鞏固寶寶免疫力,從而減少過敏反應,延續母愛級守護!

配合雀巢®低敏水解蛋白pHF-W◊
經雀巢®獨特水解技術將牛奶蛋白切細,降低致敏性7,有助減低寶寶敏感風險,糅合「免疫加乘組合」為寶寶提供免疫低敏雙重守護。

更多成分及包裝資訊
升級雀巢®能恩全護®INFINIPRO®配方昇華母源免疫精粹 HMOs (母乳低聚糖),增加HMOs種類,同時提升含量168%▀,給寶寶更全面免疫守護1-14 。
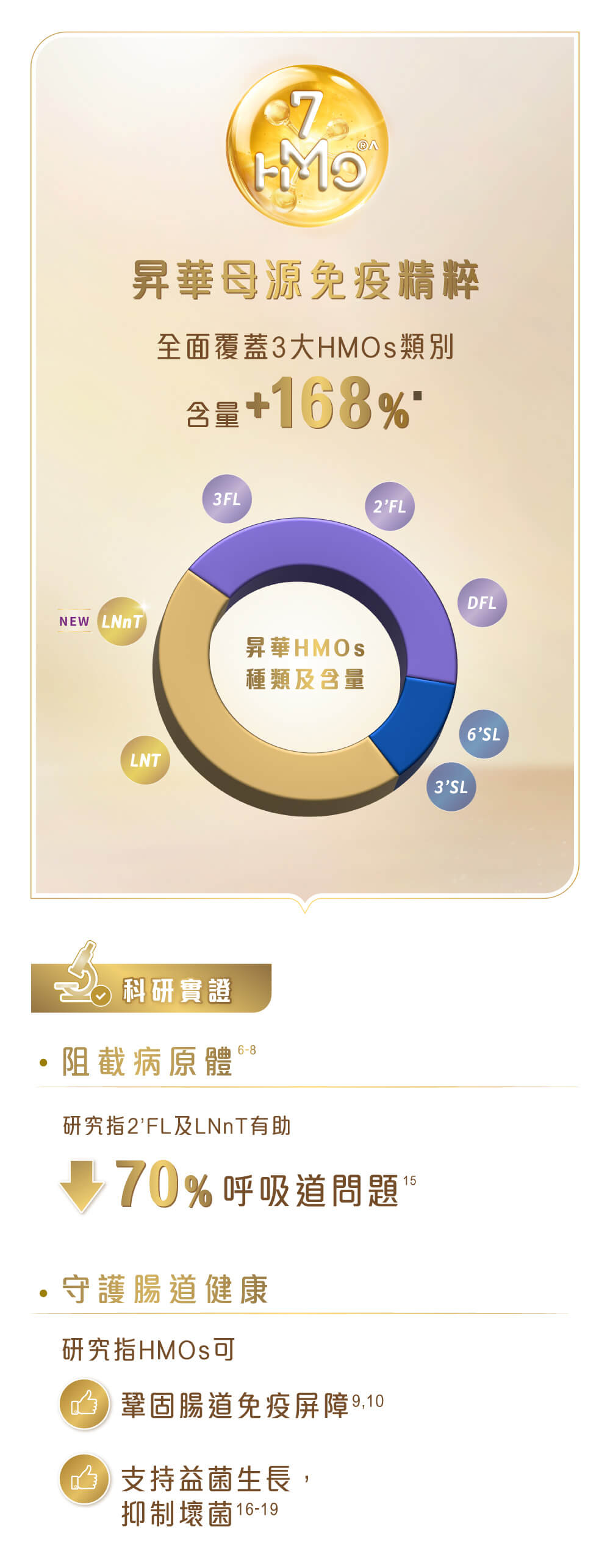
想極緻母愛免疫全護力,單靠昇華HMOs多樣性及含量並未足夠。升級雀巢®能恩全護®INFINIPRO®配方融匯專利成分*,昇華母源免疫精粹加乘星級雙益生菌+,組成「免疫加乘組合」,科研實證3大免疫加乘功效。配合低敏水解蛋白pHF-W◊,以科研延續媽媽的LOVE SCIENCE,進一步倍放低敏全護力!
▀與前配方相比,每100毫升新雀巢®能恩全護®INFINIPRO®配方4號沖調液的母乳低聚糖HMO總含量增加168%。^2’FL、3FL、LNnT、LNT、6’SL、3’SL及DFL (均屬於母乳低聚糖HMO類別,非源自母乳) 每100毫升沖調液含217毫克。*專利應用於含有6HMOs▲、B. infantis及 B. lactis 益生菌之嬰幼兒兒童產品。▲6HMOs指2‘FL、LNT、3FL、6’SL、3’SL和DFL (均屬於母乳低聚糖HMO類別,非源自母乳) 。◊與完整牛奶蛋白對比,經水解的蛋白致敏性更低。+雙益生菌指 B. infantis 及 B. lactis。1. Laucirica D R, et al. J Nutr. 2017. 147(9):1709-1714. 2. Duska-McEwen G, et al. Food Nutr Sci. 2014. 5:1387-98. 3. He S, et al.Gut. 2016. 65(1):33-46. 4. Storm H M, et al. Global Pediatric Health. 2019. 6:1-10. 5. Rochat F, et al. Abstract presented at: 6th WCPGHAN. 2020c. Copenhagen, Denmark. 6. Cravioto A, et al. J Infect Dis. 1991. 163(6):1247-55. 7. Lin A E, et al. J Biol Chem. 2017. 292(27):11243-11249. 8. Kim J, et al. Infect Immun. 2019. 87(1), e00694-18. 9. Yu Z T, et al. J Nutr. 2016. 146(10):1980-90. 10. Ruiz-Moyano S, et al. Appl Environ Microbiol. 2013. 79(19):6040-9. 11. Kavanaugh D W, et al. PLoS One. 2013. 8(6). 12. Goehring K C, et al. J Nutr. 2016. 146(12):2559-66. 13. Newburg D S. J Thromb Thrombolys. 2016b. 42(1):46-55. 14. Allen J M, et al. Front Immunol. 2019. 10. 1774. 15. Puccio G, et al. J Pediatr Gastroenterol Nutr 2017;64(4):624-31. 16. German J B, et al. Nestle Nutr Workshop Ser Pediatr Program. 2008; 62: 205-222. 17. Sela D A, et al. Proc Natl Acad Sci USA. 2008;105(48):18964-18969. 18. Marcobal A, et al. Cell Host Microbe. 2011; 10(5): 507–514. 19. Lin A E, et al. J Biol Chem. 2017; 292(27):11243-11249.
母乳是最好的。
升級雀巢®能恩全護®INFINIPRO®配方優選2款雙歧益生菌 (B. I. 及 B. L. 益生菌)。雙歧益生菌是初生寶寶腸道內最大的菌群家族1,當中以B.I. 益菌的含量最高2**。
每一種雙歧益生菌的功效都不同,研究指寶寶早期補充足量及多元的雙歧益生菌可更好支持免疫力3-4,建立強壯的健康基礎!

星級雙益生菌+加乘母源免疫精粹7HMOs^,配合低敏水解蛋白pHF-W◊ ,進一步倍放低敏全護力!
** 0-6個月的寶寶。◣與對照組比較,剖腹出世的寶寶在接受疫苗後的反應。►與對照組比較。+雙益生菌指 B. infantis 及 B. lactis。^2’FL、3FL、LNnT、LNT、6’SL、3’SL及DFL (均屬於母乳低聚糖HMO類別,非源自母乳) 每100毫升沖調液含217毫克。◊與完整牛奶蛋白對比,經水解的蛋白致敏性更低。1. Stewart C, et al. Nature. 2018. 562:583-588. 2. Vatanen et al. 2022. Cell 185:1-18. 3. Saturio S, et al. Int J Mol Sci. 2021. 22(7): 3382. 4. Dogra S.K. et al. Microorganisms. 2021.9.2110. 5. Holscher H D, et al. JPEN. 2012. 36(1):106S-117S. 6. Weizman Z et al.Pediatrics. 2005;115:5-9.
母乳是最好的。
推行可回收包裝設計 與寶寶共同孕育可持續的未來
雀巢®能恩®負起重任,雀巢®能恩全護®INFINIPRO®配方為支持地球可持續發展,除了把包裝中的鐵罐和鋁箔設計為可回收利用,更採用植物性塑料包装設計其罐蓋及量匙,替代採用來自於傳統石化燃料和其他非再生的包裝材料,以至所有的包裝物料可全部回收再生!
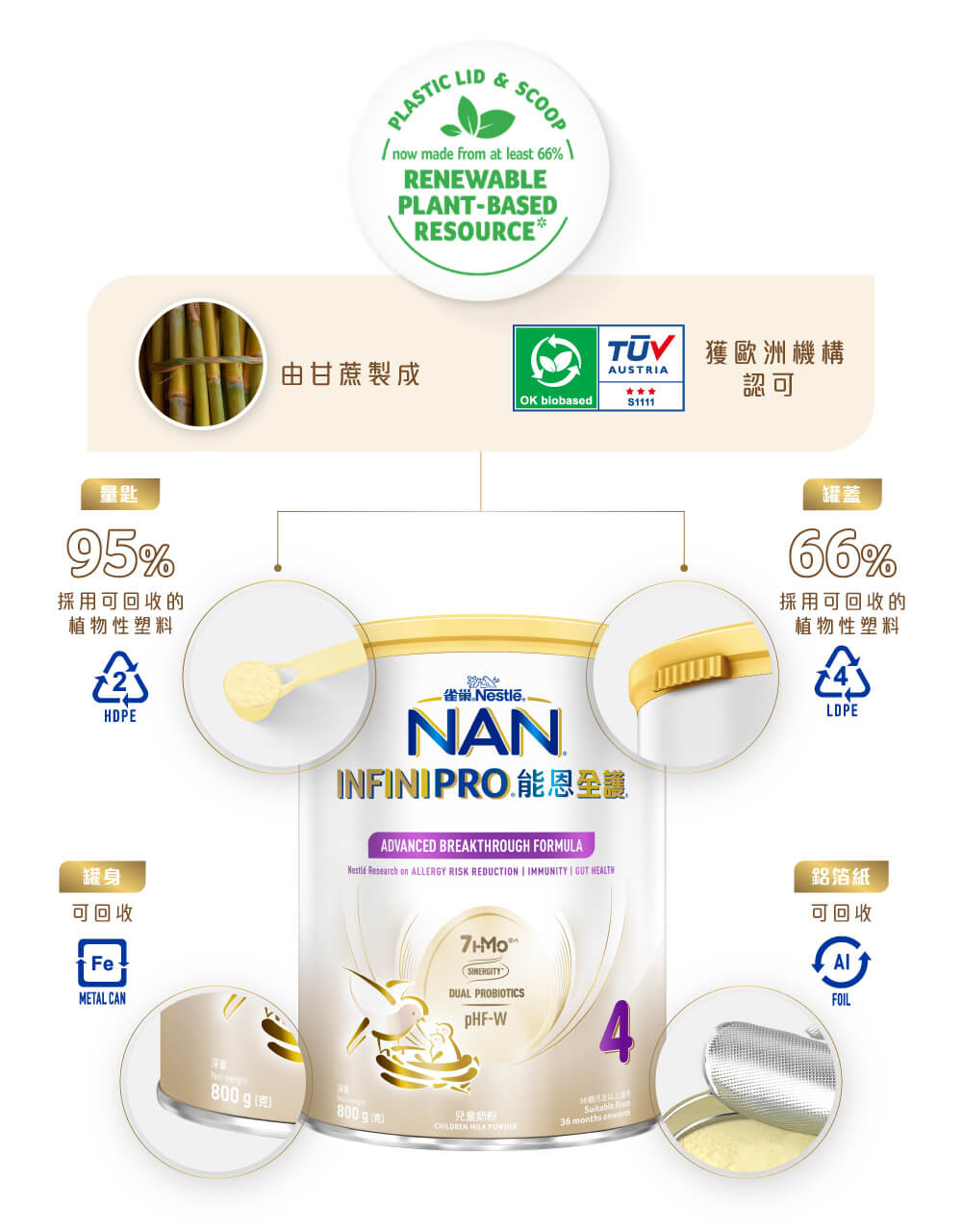
植物性塑料獲TUV Austria##認可生產罐蓋及量匙,包裝材料的外觀、功能與傳統罐蓋及量匙相同,但所用資源更少,為寶寶孕育可持續發展的未來。
雀巢®能恩全護®配方的量匙屬於HDPE 2號塑膠,可於香港政府的回收箱作分類回收。而罐蓋屬於LDPE 4號塑膠,可以把它們交到其他指定地點回收。您亦可以參考環保署「綠在區區」Waste Reduction的專頁,找出最方便你的回收點!
讓我們一起為寶寶好好守護環境,奠定未來成長銳力,盡現無盡可能!
## TUV Austria是第三方認證機構,它根據各種國際標準評估和確認我們植物性塑料包裝,並提供了與我們的蓋子和勺子的生物基含量相關的認證。⬛罐蓋及量匙內的塑膠之原材料有至少66%源自於甘蔗。
升級雀巢®能恩全護®INFINIPRO®配方是否只適合有敏感問題的寶寶?
閃耀升級雀巢®能恩全護®INFINIPRO®配方適合一般健康寶寶持續飲用。
升級配方昇華母源免疫精粹7HMOs^,加乘星級雙益生菌+,組成「免疫加乘組合」,科研實證3大免疫加乘功效,配合低敏水解蛋白pHF-W,進一步倍放低敏全護力。
升級雀巢®能恩全護®INFINIPRO®配方有配方奶即飲裝 (水奶) 嗎?
了解更多雀巢®能恩全護®INFINIPRO®配方奶即飲裝(水奶) 資訊 請按此
寶寶轉飲升級配方有什麼需要留意?
建議媽媽可以按轉奶參考表逐步為寶寶轉飲升級雀巢®能恩全護®INFINIPRO®配方。
了解更多轉奶詳情 請按此
^2’FL、3FL、LNnT、LNT、6’SL、3’SL及DFL (均屬於母乳低聚糖HMO類別,非源自母乳) 每100毫升沖調液含217毫克。
+雙益生菌指 B. infantis 及 B. lactis。

各階段配方資訊
*專利應用於含有6HMOs▲ 、B. infantis 及 B. lactis 益生菌之嬰幼兒兒童產品。
^2’FL、3FL、LNnT、LNT、6’SL、3’SL及DFL (均屬於母乳低聚糖HMO類別,非源自母乳) 每100毫升沖調液含217毫克。
▲ 6HMOs指2‘FL、LNT、3FL、6’SL、3’SL和DFL (均屬於母乳低聚糖HMO 類別,非源自母乳) 。
◄指寶寶於飲用含專利成分*的水解配方3個月後,益菌佔比 (雙歧益生菌)及壞菌佔比 (難辨梭菌) 與飲用水解配方的寶寶對比更貼近母乳組別。
#珍貴免疫營養指SCFA短鏈脂肪酸。科研實證,0-6個月寶寶於飲用同時含多元HMOs母乳低聚糖、B. infantis 及 B. lactis 益生菌的配方後,SCFA短鏈脂肪酸提升幅度達2倍;與飲用只含B. infantis 益生菌的配方的寶寶對比。
◈敏感反應指Th2免疫反應。調節Th1及Th2免疫平衡有助減低敏感免疫反應。
▵以母乳組別為基準,寶寶於服用含專利成分*的水解配方3個月後, 雙歧益菌佔比中位數與對照組相比高30.4%。
▪以母乳組別為基準,寶寶於服用含專利成分*的水解配方3個月後,平均壞菌佔比與對照組相比減低57.7%。
+理想水平為母乳組別水平。
✦指免疫T細胞。
▼理想水平為母乳組別的Th1及Th2水平。
⑅敏感相關免疫反應指Th2免疫反應。以母乳組別為基準,寶寶於服用含專利成分*的水解配方3個月後,Th2免疫反應中位數與對照組相比減低15.8%。
◊與完整牛奶蛋白對比,經水解的蛋白致敏性更低。
1. Picaud J C, et al. Front. Nutr. 2025. 12:1628847.
2. De Bruyn F, et al. Communications Biology. 2024. 7. 943.
3. Noti M, et al. In-Abstracts of the 56th ESPGHAN Annual Meeting. 2024. 5-S1401.
4. Senna G, et al. Multidisciplinary Respiratory Medicine. 2022. 17. 813.
5. Stewart C, et al. Nature. 2018. 562:583-588.
6. Alessandri G, et al. Front Immunol. 2019. 10. 2348.
7. Vandenplas Y, et al. Nutrients. 2022, 14, 1720.
母乳是最好的。




